Abstract
Background
Mature T-cell lymphomas (TCL) are a rare and aggressive group of non-Hodgkin lymphomas with significant geographic variability. Data on TCL patient outcomes by race are limited, and minority populations are often underrepresented in TCL clinical trials in North America and Europe. Herein, we describe outcomes in patients with newly diagnosed TCL from an urban US tertiary care center based on race.
Methods
We retrospectively identified 125 patients with non-cutaneous and non-leukemic mature TCL diagnosed between 1/1/2009-12/31/2021 from the electronic medical records at the Hospital of the University of Pennsylvania. In a univariate model stratified by self-reported race, we compared baseline patient characteristics at initial presentation, treatment patterns (including first line (1L) therapy, use of CNS prophylaxis and hematopoietic cell transplant (HCT), number of subsequent lines of treatment), and disease outcomes. Multivariate Cox proportional hazards models controlling for race, sex, age, ECOG score, LDH level, initial stage of disease, presence of B symptoms, hemoglobin, platelet count, IPI score, number of sites of extranodal involvement (ENI), histology, and 1L treatments (CHOP, CHOEP, other intensive treatment, radiation only, as well as palliative care), and receipt of HCT were implemented to further investigate the impact of these clinical parameters on overall survival (OS) and progression-free survival (PFS). P-values <0.05 were considered significant. Hazard ratio (HR) is reported with 95% CI in brackets.
Results
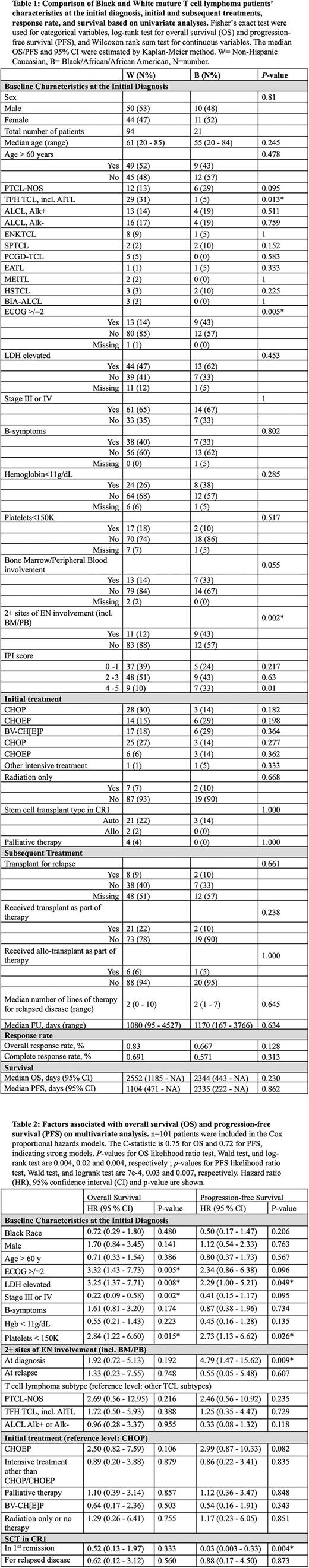
We collected and evaluated data from 94 White patients, 21 Black patients and 10 patients of other races. Patients of other races comprised less than 10% of our cohort and were omitted from the analysis due to inadequate statistical representation. Compared to White patients, Black patients demonstrated comparable baseline characteristics at initial presentation, except for lower incidence of TCL with T-follicular helper phenotype (p=0.013), poor performance status (ECOG ≥ 2; p=0.005), and ≥2 sites of ENI (p=0.002). Treatment patterns for Black and White patients, including 1L chemotherapy, radiation therapy, CNS prophylaxis, HCT, and subsequent lines of treatment were not significantly different. On univariate analysis, response rates to initial therapy, PFS, and OS were similar for Black and White patients (Table 1). On multivariate analysis, race did not significantly impact OS (HR 0.72 [0.29-1.80], p=0.480) or PFS (HR 0.50 [0.17-1.47], p=0.206), though worse outcomes were observed with other established risk factors, including ECOG ≥ 2 (OS HR 3.32 [1.43-7.73], p=0.005), elevated LDH (OS HR 3.25 [1.37-7.71], p=0.008; PFS HR 2.29 [1.00-5.21], p=0.0.049), and low platelet count (OS HR=2.84 [1.22-6.60], p=0.015; PFS HR 2.73 [1.13-6.62], p=0.026). However, advanced stage was associated with better OS (HR 0.22 [0.09 -0.58], p=0.002). For both races, HCT in 1st remission was associated with significantly improved PFS (HR 0.03 [0.03-0.33], p=0.004) but not OS (HR 0.57 [0.14-2.35] p=0.44) (Table 2).
Conclusion
In this cohort of patients with TCL treated at an urban US tertiary care center with demographics reflective of the national population, we did not find outcomes affected by race despite the higher incidence of poor risk baseline prognostic factors in Black patients. Treatment patterns, including intensive therapy, use of transplant, and number of subsequent therapies, were also not affected by race in our cohort.
Disclosures
Chong:Tessa: Consultancy; Beigene: Consultancy; KITE: Consultancy; Novartis: Consultancy; Juno/BMS: Consultancy. Gerson:Loxo Oncology: Research Funding; Abbvie: Consultancy; Genentech: Consultancy. Landsburg:Calithera: Membership on an entity's Board of Directors or advisory committees; Curis, Inc: Research Funding; Epizyme: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Triphase: Research Funding. Svoboda:TG: Research Funding; SEAGEN: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Merck: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Consultancy; BMS: Consultancy, Research Funding; Atara: Consultancy; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADCT: Consultancy. Schuster:AbbVie: Research Funding; Adaptive Biotechnologies: Research Funding; AstraZeneca: Consultancy; BeiGene: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; DTRM: Research Funding; Fate Therapeutics: Consultancy; Genentech: Consultancy, Research Funding; Genmab: Consultancy; Incyte: Consultancy, Research Funding; Juno Therapeutics: Consultancy, Research Funding; Legend Biotech: Consultancy; Loxo Oncology: Consultancy; Merck: Research Funding; MorphoSys: Consultancy; Mustang Biotech: Consultancy; Nordic Nanovector: Consultancy; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Pharmacyclics: Research Funding; Regeneron: Consultancy; Roche: Consultancy, Research Funding; TG Therapeutics: Research Funding. Barta:Daiichi Sankyo: Consultancy; Kyowa Kirin: Consultancy, Honoraria; Seagen: Honoraria; Affimed: Consultancy; Janssen: Other: Independent Data Monitoring Committee member; Acrotech: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal